The various metallurgical phenomena involved in welding — melting, freezing, diffusion, precipitation, solid-state transformations, thermal strains and shrinkage stresses — can raise many practical concerns in welded metals. These problems can be addressed by applying the appropriate metallurgical principles to the welding process.
Welding metallurgy differs from conventional metallurgy in certain important respects. However, a broad knowledge of physical metallurgy is necessary to understand welding metallurgy.
Physical metallurgy
The field of physical metallurgy relates to the study of the structure of metals and their properties, including strength, ductility, toughness and corrosion resistance.
Structure of metals
Most solid metals have a crystalline structure — the atoms composing each crystal are arranged in a specific geometric pattern that repeats throughout the metal. This orderly arrangement of the atoms, termed a lattice, forms distinguishable regions within the metal, termed a solid phase, which is responsible for many properties of metals. The most common crystalline structures found in pure metals are the face- and body-centered cubic (Figures 1A and 2B) as well as hexagonal close-packed lattices (Figure 1C). Pure metals have a single phase at any given temperature, while alloys may have more than one.
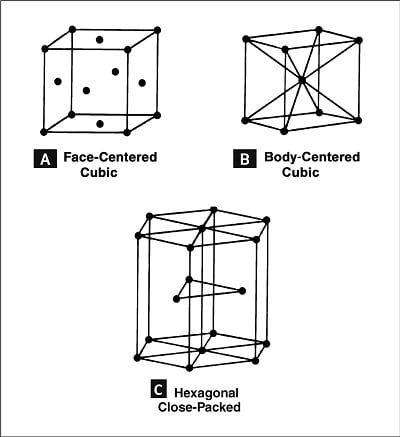 Figure 1: The three most common crystalline structures in metal.
Figure 1: The three most common crystalline structures in metal.
In the liquid state, the atoms composing metals have no orderly arrangement (there is no lattice). The liquid metal is termed amorphous, meaning that the atoms do not follow a periodic spatial arrangement, like water or glass. As the liquid metal approaches the solidification temperature, solid particles known as nuclei (initial crystal sites) begin to form at preferred sites, as shown in Figure 2A.
As shown in Figure 2B, solidification proceeds as the individual nuclei grow into larger, solid regions called grains. As the amount of solid metal increases, the amount of liquid metal decreases proportionately until the grains grow so large that there is no liquid between them. Solidification is complete at this point. As illustrated in Figure 2C, the grains meet at irregular boundaries, which are referred to as grain boundaries.
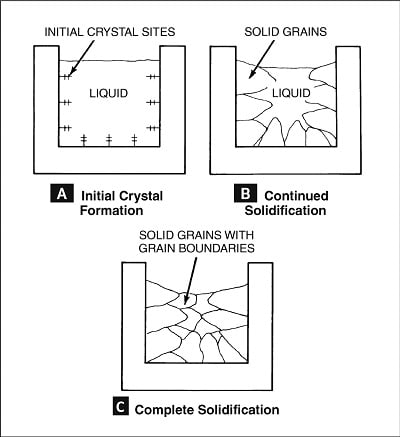 Figure 2: The solidification of a metal.
Figure 2: The solidification of a metal.
At any given temperature, each grain in a pure metal has the same crystalline structure and the same atomic spacing as all other grains. However, each grain grows independently, and the lattice orientation of the grain differs from one grain to another. The periodic and orderly arrangement of the atoms is disrupted where the grains meet, forming grain boundaries. These grain boundaries form a continuous network throughout the metal. The mechanical properties of metals are often dependent upon the size of the grains, the orientation of individual grains and the composition of the metal.
Excerpted from the Welding Handbook, Tenth Edition, Volume 1, Welding and Cutting Science and Technology.
聯(lián)系人:Rob
微信:18666076565
手 機:18925189619 (In Mandarin, Cantonese & English)
電 話:020-31190575
郵 箱:sales@gzyuto.com
公 司:廣州粵拓機電設備有限公司
地 址:廣州市番禺區(qū)橋南街蜆涌村市南路653號102
網(wǎng)站: i-campus.cn